Who We Are
Austrianni is a biotech company with the mission to develop diagnostics and therapeutics for the prevention and treatment of tuberculosis and other diseases of worldwide importance.
The company was established by the founders of Trianni, the Californian technology company that developed the Trianni human antibody platform and licensed it globally. Using this platform, Austrianni develops human therapeutic antibodies against Mycobacterium tuberculosis (Mtb) and pathogenic E. coli. Antibodies to some of the bacterial targets are also being developed as diagnostics.
The Health
Problem We Will
Help to Solve
Tuberculosis

Imagine taking a bus. Across from you, a man is coughing frequently—he might have tuberculosis (TB). If so, he could spread the infection through tiny airborne droplets released by coughing or sneezing. When inhaled, these droplets can carry Mycobacterium tuberculosis into your lungs. Your immune system may fight it , but if the bacteria establish an infection, you join the one-quarter of the world’s population carrying TB.
Tuberculosis 1/4
Most infected people never develop symptoms—only 1 in 10 cases progress to active TB. In its latent stage, the bacteria remain contained in immune cell clusters called granulomas, often detected only by chance during medical scans. However, if these granulomas break down—due to a weakened immune system or unknown reasons—TB can become active. Every year, 10 million people worldwide develop the disease.
Tuberculosis 2/4

TB is treatable, but the long antibiotic course (4–9 months) has side effects that may lead to incomplete treatment, increasing the risk of drug resistance. About 5% of TB cases are multidrug-resistant (MDR-TB), affecting 400,000 people annually. If the bacteria resist core TB drugs (extensively drug-resistant TB), treatment becomes even harder, requiring quarantine and costly care.
Tuberculosis 3/4
Austrianni is tackling this challenge with antibody-based therapies targeting multiple bacterial components. Learn more about our innovative approach on our Technology and Diagnostics pages.
Tuberculosis 4/4
Infections caused by
pathogenic Escherichia coli
We coexist with 40 trillion bacteria, mostly in our colon. About 0.1% are E. coli, which aid digestion, prevent harmful bacteria from growing, and produce vitamin K. However, when E. coli spreads to the wrong place, it can cause infections responsible for 80–95% of urinary tract infections.
E. coli infections 1/4
Some E. coli strains live in the guts of cows, sheep, and goats. While harmless to the animals, these bacteria can cause serious illness in humans, leading to abdominal pain, bloody diarrhea, kidney disease, and sometimes death. In some cases, infection can lead to hemolytic uremic syndrome, the leading cause of acute kidney failure in children in Europe and the Americas. Once infected, there are limited treatment options to prevent the syndrome’s development.
E. coli infections 2/4

Some E. coli bacteria, normally harmless in the gut, can cause urinary tract infections, nephritis, and sepsis. A few E. coli clones, spread worldwide, are responsible for most urinary tract and bloodstream infections. One clone, recently emerged as a major threat, is now a “critical priority pathogen” due to its rapid spread and multidrug resistance, a trait shared by many other dangerous pathogens.
E. coli infections 3/4

Antimicrobial resistance threatens modern medicine, making infections harder to treat and procedures like surgery, C-sections, and chemotherapy, riskier. At Austrianni, we develop antibodies as an alternative to antibiotics to fight multidrug-resistant pathogens.
E. coli infections 4/4
Diagnostics

When we visit a doctor, we want to know what’s causing our illness— whether it’s a viral or bacterial infection. Large hospitals have labs for this, but what about doctors with limited resources or even people testing themselves? The COVID-19 pandemic introduced us to „Lateral Flow Assays,“ like pregnancy tests. These tests use a few drops of urine and a red line, made of colloidal gold particles attached to antibodies, to show the result.
Diagnostics 1/3

Point-of-care tests are done at the site of care—home, doctor’s practice, or hospital—ensuring prompt action. In cases like sepsis, they can be lifesaving. For public health, these tests reduce infection spread by encouraging self-isolation. Quick, accurate TB detection helps curb its spread and supports the WHO’s goal to reduce TB deaths by 95% and incidence by 90% by 2035.
Diagnostics 2/3

For TB testing, point-of-care tests must be simple and affordable. At Austrianni, we’ve created the world’s largest collection of monoclonal antibodies targeting Mycobacterium tuberculosis antigens. Using antibodies against secreted proteins, we’re developing fast, reliable diagnostics for point-of-care and clinical testing.
Diagnostics 3/3
Management
Gloria Esposito, Ph.D.
Chief Executive Officer
Dr. Esposito obtained her Ph.D. in Genetics and Molecular Biology at the La Sapienza University of Rome. During this period she worked extensively in antibody engineering and on the development of therapeutic

Matthias Wabl, Ph.D.
Chief Scientific Officer and Acting Chairman
Dr. Wabl was a co-founder of Sagres Discovery, Inc. (now Novartis), where he served as President and as Chair of the Scientific Advisory Board, and most recently he was the Chief Executive Officer and Board Chairman of
Science Advisors
Werner Müller, Ph.D.
Dr. Müller received his Ph.D. from the University of Cologne, where he was a pioneer in generating countless transgenic mice now used all over the World. He was one of the founding members of the IMGT database, which
Michel Streuli, Ph.D.
Dr. Michel Streuli is Chief Executive Officer of Foundery Innovations, an immunotherapy venture studio. Prior to Foundery, he was Senior Vice President and Chief Scientific Officer of Pionyr Immunotherapeutics, Inc.
Prof. Sir Stewart Thomas Cole KCMG FRS
Professor Stewart Cole is an internationally renowned microbiologist working in global health. He has made outstanding contributions to HIV and HPV genomics, antimicrobial resistance research
Prof. Markus Zeitlinger
Gloria Esposito, Ph.D.
Chief Executive Officer
Dr. Esposito obtained her Ph.D. in Genetics and Molecular Biology at the University of Rome “La Sapienza”. During this period she worked extensively in antibody engineering and on the development of therapeutic antibodies against viral infections. She completed her postdoctoral training in the Laboratory of Klaus Rajewsky in Cologne (Germany), where she subsequently held a position as Investigator. The major focus of her research was on lymphocyte development and antibody maturation. She moved from academia to the Dutch pharmaceutical company Organon, where she led an in vivo drug target validation group, and then to Taconic, where she established, and for many years led, the Scientific Project Management and Sales Team with global responsibility for the Custom Model Generation portfolio.
Matthias Wabl, Ph.D.
Chief Scientific Officer and Acting Chairman
Dr. Wabl was a co-founder of Sagres Discovery, Inc. (now Novartis), where he served as President and as Chair, Scientific Advisory Board. Most recently, he has been the Chief Executive Officer and Board Chairman of Trianni Inc. (trianni.com; now an AbCellera Company), which he founded. He was a member of the NIH, Small Business Innovative Research Study Section, and an advisor to numerous biotechnology companies and to the FDA. He is also a Professor of Microbiology and Immunology at the University of California at San Francisco (UCSF) where he has been engaged in research on the generation of antibody diversity and the basis of autoimmunity for over 30 years. He received his engineering degree in chemistry from the Technical University in Graz and his Ph.D. in biology from the Max-Planck Institute in Berlin. He was a Member of the Basel Institute for Immunology and a Principal Investigator at the Max-Planck Institute in Tübingen, Germany.
Werner Müller, Ph.D.
Dr. Müller received his Ph.D. from the University of Cologne, where he was a pioneer in generating countless transgenic mice now used all over the World. He was one of the founding members of the IMGT database, which contains sequences encoding antibody, T-cell receptor and histocompatibility genes. Using advanced mutant mice, he showed that Interleukin-4 is critical for an effective antibody response against parasite infections. At the Helmholtz Centre of Infection Research in Braunschweig, he established the first European “mouse infection challenge platform” as part of the European Mouse Disease Clinic. This platform facilitates the study of gene functions in bacterial and parasite infections. Currently, Dr. Müller holds the Bill Ford Chair of Cellular Immunology at the University of Manchester, UK, where he continues to study the role of cytokines on inflammation and host defense in parasite infection.
Michel Streuli, Ph.D.
Michel Streuli is Chief Executive Officer of Foundery Innovations, an immunotherapy venture studio. Prior to Foundery, he was Senior Vice President and Chief Scientific Officer of Pionyr Immunotherapeutics, Inc. Michel held senior research positions at Gilead, Merck, Schering-Plough, and Organon, following a decade on the faculties of the Dana-Farber Cancer Institute and Harvard Medical School.
During his 20+ years in the pharmaceutical and biotech industry, he has led numerous drug development programs from discovery through the early clinical stage, with a focus on therapies for cancer, chronic viral, autoimmune, and inflammatory diseases, including overseeing the development of the immunomodulatory anti-PD-1 monoclonal antibody Keytruda, and chairing the Keytruda early development team that brought Keytruda to the clinic.
Michel holds a Ph.D. degree from the University of Zurich, where he was involved in the cloning and characterization of interferon-alpha-2, which led to the development of INTRON A and PEG-INTRON.
Dr. Alfred Gusenbauer
Director
Dr. Alfred Gusenbauer served as Federal Chancellor of Austria and is a member of the Club de Madrid, an independent organization of more than 80 former presidents and prime ministers. He remains advisor to many heads of state. Mr. Gusenbauer is the first Leitner Global Fellow at the Columbia University School of International and Public Affairs in New York. He is also Head of the Supervisory Board of Strabag, Austria’s leading construction company, and he serves on the board of several other companies.
Andreas Wabl
Trustee
Andreas Wabl was a Co-Founder of the Austrian Green Party, the Chairman of the Austrian Government Accountability Office, and a Member of the Austrian Parliament for 13 years. He also served as the Climate Commissioner of the Federal Chancellor of Austria. Mr. Wabl studied at the University College of Teacher Education Carinthia and is a licensed mediator.
Prof. Sir Stewart Thomas Cole KCMG FRS
Professor Stewart Cole is an internationally renowned microbiologist working in global health.
He has made outstanding contributions to HIV and HPV genomics, antimicrobial resistance
research and the molecular microbiology of toxigenic clostridia. However, he is most highly
acclaimed for his pioneering work on the pathogenicity, evolution and genomics of the
mycobacteria responsible for tuberculosis (TB) and leprosy. His team harnessed genome-
derived information to accelerate TB drug and vaccine discovery and development, and
candidate drugs that arose from his work are currently in clinical trials. Throughout his career
he has strived to translate findings from his discovery research into interventions that benefit
human health. Professor Cole is a past president of the Institut Pasteur, Paris, and the Global
Health Institute at the Swiss Federal Institute of Technology in Lausanne.
Prof. Markus Zeitlinger
Matthias Wabl, Ph.D.
Chief Scientific Officer and Acting Chairman
Dr. Wabl was a co-founder of Sagres Discovery, Inc. (now Novartis), where he served as President and as Chair, Scientific Advisory Board, and most recently he has been the Chief Executive Officer and Board Chairman of Trianni Inc (trianni.com). He has been a member of the NIH, Small Business Innovative Research Study Section and an advisor to numerous biotechnology companies and the FDA. He is also a Professor of Microbiology and Immunology at the University of California at San Francisco (UCSF) where he has been engaged in research on the generation of antibody diversity and the basis of autoimmunity for over 20 years. He received his engineering degree in chemistry from the Technical University in Graz and his Ph.D. in biology from the Max-Planck Institute in Berlin. He was a Member of the Basel Institute for Immunology and a Principal Investigator at the Max-Planck Institute in Tübingen, Germany.
Our Team
In our quest to combat tuberculosis, we have assembled an enthusiastic multinational team from Austria, Cameroon, Germany, Italy, Portugal, Serbia, and the United States.
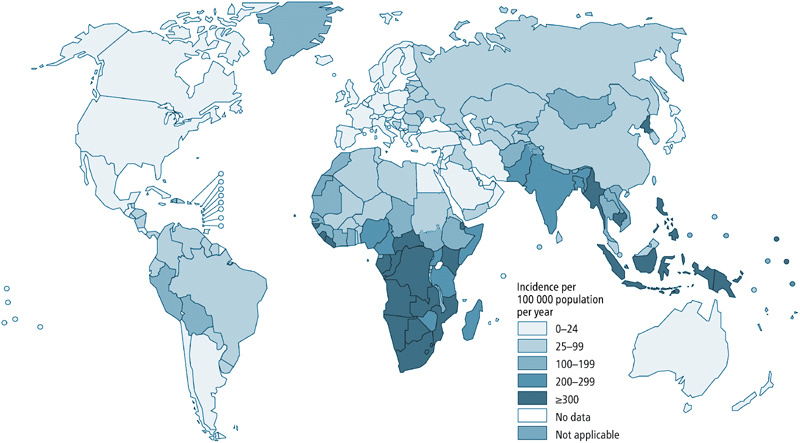
World TB Incidence
Tuberculosis (TB) kills more than a million people each year and incapacitates ten times more. One quarter of the world’s population is infected with TB, and TB is among the top 10 causes of death world-wide. While it is currently most prevalent in low-income countries, cases arise everywhere. Adding to the health crisis, drug-resistant forms of the disease emerge as a major threat especially in Central Asia and Eastern Europe, but they may become even more widespread.
© 2025 AUSTRIANNI. All rights reserved.
![VBC_Co-Branding_Logo[84][30] (1) 11](https://www.austrianni.com/wp-content/uploads/2025/04/VBC_Co-Branding_Logo8430-1-11.png)